MEDIUM
JEE Main
IMPORTANT
Earn 100
Low oxidation state of metals in their complexes are common when ligands
(a)have good -accepting character
(b)have good -donor character
(c)are havind good -donating ability
(d)are havind poor -donating ability
33.33% studentsanswered this correctly

Important Questions on Coordination Compounds
HARD
JEE Main
IMPORTANT
Total number of relatively more stable isomer(s) possible for octahedral complex will be____
HARD
JEE Main
IMPORTANT
Match List-I with List-II
| List-I (Complex) |
List-II (Hybridization) |
||
| A | I | ||
| B | II | ||
| C | III | ||
| D | IV |
Choose the correct answer from the options given below
HARD
JEE Main
IMPORTANT
should be an inner orbital complex. Ignoring the pairing energy, the value of crystal field stabilization energy for this complex is _____.
HARD
JEE Main
IMPORTANT
Octahedral complexes of copper undergo structural distortion (Jahn-Teller). Which one of the given copper complexes will show the maximum structural distortion?
(en-ethylenediamine; )
EASY
JEE Main
IMPORTANT
Sum of oxidation state (magnitude) and coordination number of cobalt in is
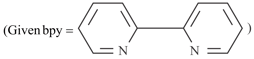
EASY
JEE Main
IMPORTANT
The primary and secondary valencies of cobalt respectively in are:
MEDIUM
JEE Main
IMPORTANT
The d-electronic configuration of in tetrahedral crystal field is . The sum of and number of unpaired electrons is
EASY
JEE Main
IMPORTANT
Which of the following cannot be explained by crystal field theory?
