Magnesium is extracted by the electrolysis of molten magnesium chloride.
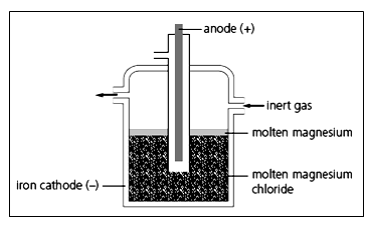
What information in the diagram suggests that magnesium is less dense than molten magnesium chloride?


Important Questions on Cambridge IGCSE Exam Questions from Paper 2
Magnesium is extracted by the electrolysis of molten magnesium chloride.
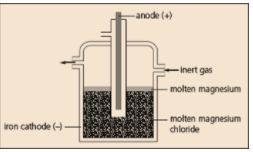
Magnesium is extracted by electrolysis rather than by heating its oxide with carbon. Why?
Magnesium is extracted by the electrolysis of molten magnesium chloride.
Suggest why a stream of inert gas is blown over the surface of the molten magnesium.
Magnesium is extracted by the electrolysis of molten magnesium chloride.
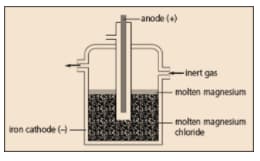
Name a gaseous element which is inert.
In some old magnesium manufacturing plants, coal gas is blown over the surface of the magnesium. The list shows the main substances in coal gas: carbon monoxide, ethene, hydrogen, hydrogen sulfide, methane. Draw the structure of ethene showing all atoms and bonds.
Carbon monoxide can be removed from coal gas by mixing it with steam and passing the mixture over a catalyst of iron oxide at .
.
Write a word equation for this reaction.
Carbon monoxide can be removed from coal gas by mixing it with steam and passing the mixture over a catalyst of iron oxide at .
.
What does the symbol mean?
Carbon monoxide can be removed from coal gas by mixing it with steam and passing the mixture over a catalyst of iron oxide at .
.
Iron oxide reacts with acids to form a solution containing iron ions. Describe a test for aqueous iron ions. Give the result.
