EASY
NEET
IMPORTANT
Earn 100
Match the compounds of Xe in column I with the molecular structure in column II.
Column-I
Column-II
(a)
(p)
Square planar
(b)
(q)
Linear
(c)
(r)
Square pyramidal
(d)
(s)
Pyramidal
(a)
(a)-(q), (b)-(p), (c)-(r), (d)-(s)
(b)(a)-(q), (b)-(s), (c)-(r), (d)-(p)
(c)(a)-(q), (b)-(r), (c)-(p), (d)-(s)
(d)(a)-(q), (b)-(p), (c)-(s), (d)-(r)
72.73% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
NEET
IMPORTANT
Identify the wrongly matched pair:
MEDIUM
NEET
IMPORTANT
The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
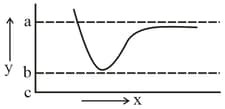
The bond energy of is :
EASY
NEET
IMPORTANT
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?
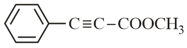
MEDIUM
NEET
IMPORTANT
Which of the following set of molecules will have zero dipole moment?
MEDIUM
NEET
IMPORTANT
Identify a molecule which does not exist.
EASY
NEET
IMPORTANT
The number of sigma and pi bonds in pent- -en- -yne is:
MEDIUM
NEET
IMPORTANT
Which of the following diatomic molecular species has only bonds according to Molecular Orbital Theory?
HARD
NEET
IMPORTANT
Which of the following is incorrect statement?
