EASY
MHT-CET
IMPORTANT
Earn 100
Match the molecules to their molecular shapes
Molecule
Molecular Shape
i.
a.
Bent
ii.
b.
Square planar
iii.
c.
Trigonal planar
iv.
d.
Sea saw
(a)i-c, ii-a, iii-b, iv-d
(b)i-a, ii-b, iii-d, iv-c
(c)i-b, ii-c, iii-d, iv-a
(d)i-c, ii-d, iii-b, iv-a
50% studentsanswered this correctly

Important Questions on Nature of Chemical Bond
EASY
MHT-CET
IMPORTANT
As the electronegativity increases from iodine to fluorine, the bond angle _____, from .
EASY
MHT-CET
IMPORTANT
The valence bond theory is unable to explain the_____
EASY
MHT-CET
IMPORTANT
Which of the following is NOT the demerit of valence bond theory?
EASY
MHT-CET
IMPORTANT
orbital of one atom do not combine with orbital of another atom because of their _____.
EASY
MHT-CET
IMPORTANT
Which of the following is CORRECT with respect to the molecular orbitals?
EASY
MHT-CET
IMPORTANT
For a stable molecule, the value of bond order should be _____
EASY
MHT-CET
IMPORTANT
The following figure represents the formation of ____ molecular orbital
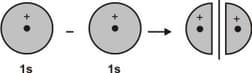
EASY
MHT-CET
IMPORTANT
The molecular orbital configuration of a diatomic molecule is
Its bond order is ____
