MEDIUM
Earn 100
Mention the conclusion of the Geiger-Marsden -particle scattering experiment.
Important Questions on Atoms
EASY
EASY
EASY
EASY
HARD
MEDIUM
EASY
EASY
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
EASY
MEDIUM
MEDIUM
MEDIUM
(Planck's constant electron mass )
EASY
MEDIUM
MEDIUM
HARD
EASY
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
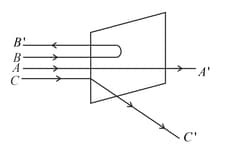
MEDIUM
HARD
EASY

