EASY
Earn 100
Metal carbonates and hydrogen carbonates react with acids to give
(a)Carbon dioxide gas
(b)Hydrogen gas
(c)Sulphur dioxide gas
(d)Carbon monoxide gas
50% studentsanswered this correctly
Important Questions on Consequences
MEDIUM
MEDIUM
MEDIUM
Write a chemical equation for the reaction taking place in the flask. Write name and one property of the gas evolved.
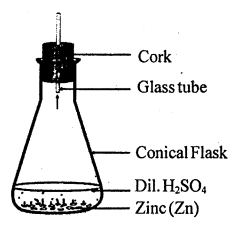
EASY
EASY
State one observation for the following:
A small piece of zinc is added to dilute hydrochloric acid.
MEDIUM
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
MEDIUM
MEDIUM
Which gas is produced during the reaction in the test tube? How does this gas react with calcium hydroxide/lime water?
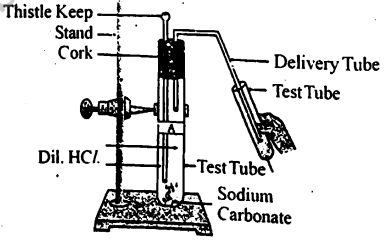
EASY
EASY
EASY
MEDIUM
State one relevant observation for of the following:
Lead nitrate solution is mixed with dilute hydrochloric acid and heated.
MEDIUM
Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
MEDIUM

