MEDIUM
Earn 100
Methyl orange gives red colour in
(a)Sodium carbonate solution
(b)Sodium chloride solution
(c)Hydrochloric acid solution
(d)Potassium hydroxide solution
50% studentsanswered this correctly
Important Questions on Equilibria
HARD
HARD
EASY
HARD
HARD
MEDIUM
MEDIUM
MEDIUM
HARD
HARD
HARD
HARD
MEDIUM
EASY
The titration curve for titration of a solution of a diprotic acid with is shown below.
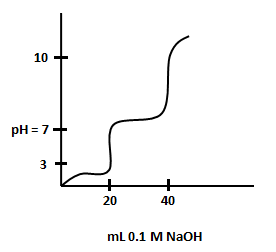
and are approximately
HARD
HARD
HARD
HARD
MEDIUM
EASY

