MEDIUM
Earn 100
Methylethylamine cannot be resolved into enantiomers because
(a)It is planar
(b)It undergoes rapid inversion
(c)It has a plane of symmetry
(d)A tricovalent atom cannot be a centre of chirality.
50% studentsanswered this correctly
Important Questions on Fundamentals of Organic Chemistry
EASY
MEDIUM
HARD
The Fischer projection of -erythrose is shown below.
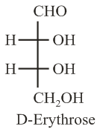
-Erythrose and its isomers are listed as and in Column I. Choose the correct relationship of and with -erythrose from Column II.
| Column - I | Column -II | ||
| P. | 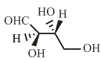 |
1. | Diastereomer |
| Q. | 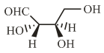 |
2. | Identical |
| R. | 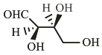 |
3. | Enantiomer |
| S. | 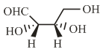 |
MEDIUM
MEDIUM
EASY
EASY
EASY
EASY
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
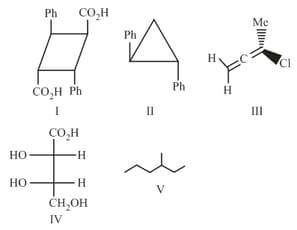
the compounds which can exhibit optical activity are :
MEDIUM
EASY
EASY
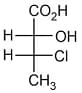 is:
is:MEDIUM
Which of the following alkenes can generate optically active compounds upon hydrogenation?
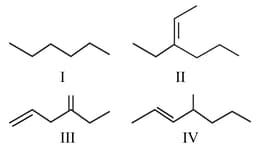
MEDIUM

