EASY
Earn 100
does not form either peroxide or superoxide, because
(a) ion is relatively bigger
(b) ion is relatively smaller
(c) ion is stable
(d) ion is unstable
50% studentsanswered this correctly
Important Questions on The s-Block Elements
EASY
MEDIUM
Reaction of with gives :
(A)
(B)
(C)
(D)
(E)
Choose the correct answer from options given below
EASY
EASY
EASY
HARD
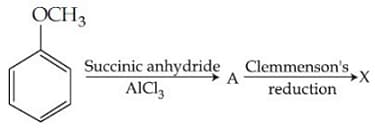
X is :
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
MEDIUM
MEDIUM
EASY
EASY
Among the statement (I – IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than AI.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds
MEDIUM
EASY
Reason (R) : Both and have almost same ionic radius
The correct option among the following is
MEDIUM
EASY
MEDIUM

