MEDIUM
Earn 100
Molar conductivity of ionic solution depends on _____.
(a)temperature.
(b)distance between electrodes.
(c)concentration of electrolytes in solution.
(d)surface area of electrodes.
50% studentsanswered this correctly
Important Questions on Electrochemistry
EASY
EASY
EASY
HARD
MEDIUM
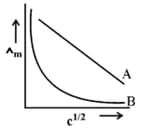
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
Answer the following :
What happens on extrapolation of to concentration approaching zero for electrolytes A and B ?
MEDIUM
Given below are two statements:
Statement I: For , molar conductivity increases steeply with dilution.
Statement II: For carbonic acid, molar conductivity increases slowly with dilution. In the light of the above statements, choose the correct answer from the options given below
MEDIUM
HARD
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
EASY
HARD
(where the constant B is positive)
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM