HARD
JEE Main/Advance
IMPORTANT
Earn 100
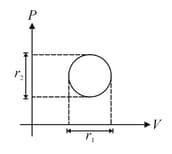
moles of a diatomic gas performs a work on its surrounding when amount of heat is supplied to the gas. Molar heat capacity of the process is . Value of , is
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main/Advance
IMPORTANT
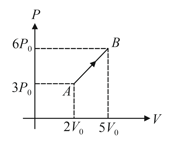
One mole of a monatomic gas undergoes the process in the given diagram. The molar specific heat for the process is . Value of , is
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
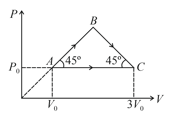
An ideal gas expands from a volume litre and pressure bar to volume litre along two different paths and as shown in figure. The heat added to the gas along the path is . The heat transfer in the process along the path is . Value of , is
HARD
JEE Main/Advance
IMPORTANT
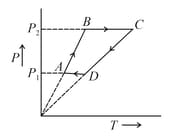
Six moles of an ideal gas performs a cycle shown in figure. If temperatures are and , the temperature at point , is . Value of , is
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
A sample of gas is going through cyclic process, work-done by gas is