EASY
JEE Main
IMPORTANT
Earn 100
required for a reaction is produced by the decomposition of in as per the equation,
The initial concentration of is and it is after 30 minutes. The rate of formation of is:
(a)
(b)
(c)
(d)
44.44% studentsanswered this correctly

Important Questions on Chemical Kinetics
MEDIUM
JEE Main
IMPORTANT
In the following reaction;
‘A’ and ‘B’ respectively can be:
EASY
JEE Main
IMPORTANT
, the rate equation for the reaction is given as
Rate
If the concentration of A is kept the same but that of B is doubled what will happen to the rate itself?
EASY
JEE Main
IMPORTANT
For the equilibrium, . If the ratio of the activation energies of the forward and reverse reactions is then:
HARD
JEE Main
IMPORTANT
For an elementary chemical reaction, , the expression for is:
HARD
JEE Main
IMPORTANT
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)
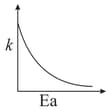
(II)
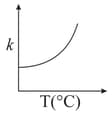
MEDIUM
JEE Main
IMPORTANT
For the reaction of with , the rate constant is at and at . The activation energy for the reaction, in is:
MEDIUM
JEE Main
IMPORTANT
A bacterial infection in an internal wound grows as , where the time is in hours. A dose of antibiotic, taken orally, needs 1 hour to reach the wound. Once it reaches there, the bacterial population goes down as . What will be the plot of vs after hour?
EASY
JEE Main
IMPORTANT
The rate law for the reaction below is given by the expression
If the concentration of is increased from , keeping the value of at , the rate constant will be:
