used to produce for use in photography it can be prepared as
If yields of reaction is and is then calculate the mass of iron required to produce
Important Questions on Some Basic Concepts of Chemistry
Atomic mass,
When of an organic compound was analyzed using Carius method for estimation of bromine, of was obtained. The percentage of bromine in the organic compound is _________.(Nearest integer)
[Atomic mass : Silver Bromine ]
If the reaction sequence given below is carried out with moles of acetylene, the amount of the product formed (in ) is
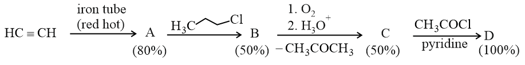
The yields of and are given in parentheses.
[Given: Atomic mass of ]
of a substance upon dissociation reaction, yields of hydrogen, of oxygen and of carbon. Given that the atomic masses of and are and , respectively. The data agrees with how many formulae of the following?
A.
B.
C.
D.
[Given : atomic mass of amu and amu]
In the following reaction
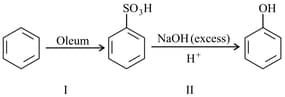
The yield for reaction is and that of reaction II is . The overall yield of the complete reaction is

