EASY
Earn 100
Name the following :
Longest period of the periodic table. (Sixth period/Fifth period)
50% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
EASY
The last element of the - block in period is represented by the outermost electronic configuration:
EASY
The elements with atomic numbers and belong to, respectively:
EASY
What will be the symbol and name for the element with atomic number ?
HARD
has a smaller first ionization enthalpy than . Consider the following statement:
(I) it is easier to remove electron than electron
(II) electron of is more shielded from the nucleus by the inner core of electrons than the electrons of
(III) electron has more penetration power than electron
(IV) atomic radius of is more than
(atomic number )
The correct statements are.
MEDIUM
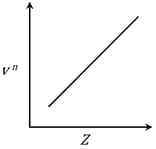
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power 'n' i.e. of X-rays emitted is plotted against atomic number , the following graph is obtained.
The value of 'n' is
EASY
Which among the following elements is a metalloid?
MEDIUM
Consider the following radioactive decays
I. and
II.
In which case group of parent and daughter elements remains unchanged.
EASY
The electronic configurations of Eu (Atomic no. ), Gd (Atomic no. ) and Tb (Atomic no. ) are:
MEDIUM
Henry Moseley studied characteristic -ray spectra of elements. The graph which represents his observation correctly is
Given Frequency of X-ray emitted
Atomic number
EASY
The IUPAC symbol for the element with atomic number would be:
EASY
How does the ionisation energy of group elements vary ?
MEDIUM
How do you appreciate the special nature of Inert gases?
MEDIUM
The first ionization energy of magnesium is smaller, as compared to that of elements and , but higher than that of . The elements and , respectively, are :
EASY
Decrease in ionic size in a period is observed in
EASY
The characteristics of elements and with atomic numbers, respectively, and are:
MEDIUM
The electronic configurations of bivalent europium and trivalent cerium are:
(atomic number: , ,
EASY
For the following Assertion and Reason, the correct option is:
Assertion: For hydrogenation reactions, the catalytic activity increases from Group to Group metals with maximum activity shown by Group elements.
Reason: The reactants are most strongly adsorbed on group elements.
HARD
What would be the IUPAC name for the element with the atomic number ?
EASY
The outermost subshell electronic configuration of an element is .
Write the atomic number of the element.
MEDIUM
Give scientific reasons:
Elements belonging to the same group have the same valency.