EASY
Earn 100
Name the following — Radioactive alkali metal.
Important Questions on s-Block Elements
MEDIUM
EASY
HARD
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
HARD
MEDIUM
MEDIUM
EASY
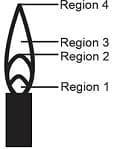
EASY
EASY

