Name the product formed by the reaction of with sodium hydroxide.
Important Questions on Inorganic Qualitative Analysis
An inorganic Lewis acid shows in the reaction:
It fumes in moist air:The intensity of fumes increases when a rod dipped in is brought near it.An acidic solution of on addition of and gives a precipitate which dissolves in solution:An acidic solution of does not give precipitate with . Identify and give chemical equations for reactions at steps to .
An inorganic Lewis acid shows in the reaction:
It fumes in moist air:The intensity of fumes increases when a rod dipped in is brought near it.An acidic solution of on addition of and gives a precipitate which dissolves in solution:An acidic solution of does not give precipitate with . Identify and give chemical equations for reactions at steps to .
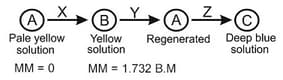
The sequential unknown reagents is / are :
Copy and complete the following table:
| Compound | Solubility in sodium Hydroxide | Solubility in Ammonium Hydroxide |
| Copper hydroxide | ||
| Aluminium hydroxide | ||
| Zinc hydroxide |
An inorganic Lewis acid shows in the reaction:
It fumes in moist air:The intensity of fumes increases when a rod dipped in is brought near it.An acidic solution of on addition of and gives a precipitate which dissolves in solution:An acidic solution of does not give precipitate with . Identify and give chemical equations for reactions at steps to .
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and

