EASY
Earn 100
Nitric oxide reacts with and gives nitrosyl bromide as per the reaction given below.
When mole of and mole of are mixed in a closed container at a constant temperature, mole of is obtained at equilibrium. Calculate the equilibrium amount of and .
(a)Moles of
Moles of
Moles of
(b)Moles of
Moles of
Moles of
(c)Moles of
Moles of
Moles of
(d)Moles of
Moles of
Moles of
50.98% studentsanswered this correctly
Important Questions on Equilibrium
HARD
(At )
EASY
Graph of a reversible process;
is given. Analyse the graph and answer the following question.
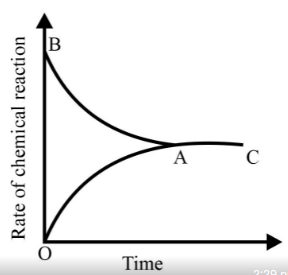
Identify the part of the graph which represents the forward reaction
[ OA, BA, AC]
MEDIUM
EASY
MEDIUM
Using the data provided, find the value of equilibrium constant for the following reaction at and atm pressure.
HARD
EASY
HARD
MEDIUM
MEDIUM
HARD
At and pressure, the degree of dissociation of is . If one mole of gas is contained in a vessel, then the density of the equilibrium mixture is
MEDIUM
EASY
EASY
.....(1)
.....(2)
The relation between and is:
HARD
For this equilibrium, the correct statement(s) is/are
EASY
(assuming ideality)
MEDIUM
The equilibrium constant () of the reaction:
, will be:
MEDIUM
Graph of a reversible process,
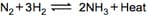
is given. Analyse the graph and answer the following question.
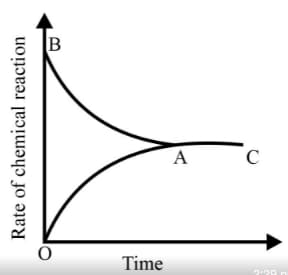
From the given statements, select the correct ones regarding chemical equilibrium.
(i0 The chemical equilibrium is 'static' at the molecular level.
(ii) Both reactants and products co-exist.
(iii) The rates of forward reaction and backward reactions are equal.
(iv) Chemical equilibrium is attained in an open system.
EASY
At equilibrium, the mass of each of the reactants and products remains constant.
At equilibrium, the rate of forward reaction is equal to the rate of backward reaction.
EASY

