Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.

Important Questions on Hydrocarbons
Nucleophiles and electrophiles are reaction intermediates having electron rich and electron deficient centres respectively. Hence, they tend to attack electron deficient and electron rich centres respectively. Identify the following species is electrophile or nucleophile.
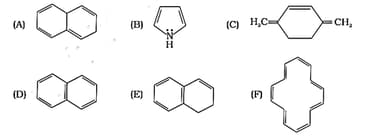
The ring systems having following characteristics are aromatic.
(i) Planar ring containing conjugated bonds.
(ii) Complete delocalisation of the -electrons in ring system i.e. each atom in the ring has unhybridised p-orbital, and
(iii) Presence of -electrons in the ring where n is an integer [Huckel rule].
Using this information classify the following compounds as aromatic/ nonaromatic.
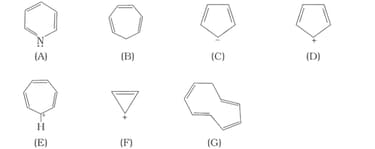
Which of the following compounds are aromatic according to Huckel’s rule?