EASY
Chemistry
IMPORTANT
Earn 100
Number of sigma bonds in is:
(a)
(b)
(c)
(d)
80% studentsanswered this correctly

Important Questions on Chemical Bonding & Molecular Structure
EASY
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
The ratio of to bonds in naphthalene is
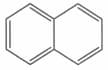
EASY
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
What is true about molecule?
It has two lone pairs.
It has one dative bond.
It has two bonds.
It has one bond.
MEDIUM
Chemistry
IMPORTANT
EASY
Chemistry
IMPORTANT
The table shown below gives the bond dissociation energies for single covalent bonds of carbon atoms with element and . Which element has the smallest atoms?
HARD
Chemistry
IMPORTANT
Consider the following statement and arrange in the order of true / false.
: In , the bonding takes place in the ground state and the bond angle is slightly less than .
: The molecular geometry of is pentagonal bipyramidal having two different bond lengths.
: In , the bond angles, instead of being and , are and respectively due to the presence of a lone pair.
