HARD
JEE Main
IMPORTANT
Earn 100
bond length in is than the bond length in . The bond length in is than that of the bond in . Choose the correct option for and from the given below.
(a) - shorter, shorter
(b)-shorter, -longer
(c) -longer, -longer
(d) -longer, - shorter
40.74% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
EASY
JEE Main
IMPORTANT
In the following molecules,
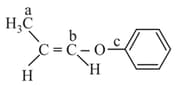
Hybridisation of carbon and respectively are :
HARD
JEE Main
IMPORTANT
The number of species below that have two lone pairs of electrons in their central atom is ___ (Round off to the Nearest integer)
MEDIUM
JEE Main
IMPORTANT
is a covalent diatomic molecule where and are second row elements of periodic table. Based on Molecular orbital theory, the bond order of is The total number of electrons in is_____. (Round off to the Nearest Integer).
EASY
JEE Main
IMPORTANT
Amongst the following, the linear species is:
EASY
JEE Main
IMPORTANT
The total number of sigma bond/s in mesityl oxide is (Round off to the Nearest Integer).
EASY
JEE Main
IMPORTANT
A central atom in a molecule has two lone pairs of electrons and forms three single bonds. The shape of this molecule is
EASY
JEE Main
IMPORTANT
Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R :
Assertion A : The bond angle in water molecule is .
Reason R : The lone pair lone pair repulsion of electrons is higher than the bond pair bond pair repulsion.
EASY
JEE Main
IMPORTANT
The number of hybrid orbitals in a molecule of benzene is:
