HARD
Chemistry
IMPORTANT
Earn 100
undergoes photochemical dissociation into a normal oxygen atom and a more energetic oxygen atom . If has more energy than and normal dissociation energy of is , what is the maximum wavelength effective for the photochemical dissociation of ?
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Structure of Atom
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
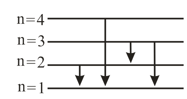
Suppose that a hypothetical atom gives a red, green, blue and violet line. Which electron jump would give off the red spectral line according to figure ?
HARD
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
Where . What is the maximum radial distance of node from nucleus?
MEDIUM
Chemistry
IMPORTANT
Consider the following statements:
Electron density in the plane in orbital is zero
Electron density in the plane in orbital is zero.
orbital has one nodal surface.
orbital, is the nodal plane.
From the above statements, which are incorrect statements?
HARD
Chemistry
IMPORTANT
