EASY
Earn 100
molecule is a resonance hybrid of the two structures and
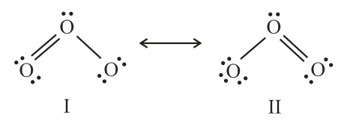
The two oxygen-oxygen bond lengths in ozone molecule are

(a)Identical
(b)Slightly different
(c)Largely different
(d)Cannot be measured due to resonance
45.45% studentsanswered this correctly
Important Questions on p- Block Elements
MEDIUM
MEDIUM
EASY
HARD
EASY
EASY
HARD
HARD
Give reason for the following statement.
Ozone is thermodynamically less stable than oxygen.
MEDIUM
EASY
EASY
EASY
EASY
acts as a powerful oxidising agent.
EASY
MEDIUM

