MEDIUM
AP EAPCET
IMPORTANT
Earn 100
Observe the following molecules.
The number of molecules having square pyramidal geometry from the above is
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
AP EAPCET
IMPORTANT
and are two covalent molecules in which the hybridization of the central atoms is the same, but the shapes are different. What are and ?
MEDIUM
AP EAPCET
IMPORTANT
In which of the following molecules/ions, the central atom is hybridized?
MEDIUM
AP EAPCET
IMPORTANT
Match the following?
| Molecule | Geometry | ||
| (a) | (i) | Angular (or) Bent | |
| (b) | (ii) | See-saw | |
| (c) | (iii) | Square pyramidal | |
| (d) | (iv) | T-shape | |
| Square planar |
MEDIUM
AP EAPCET
IMPORTANT
The correct order of electronegativity of carbon in various hybridization states:
EASY
AP EAPCET
IMPORTANT
The formation of molecular orbitals can be described by the linear combination of atomic orbitals. Which one of the following correctly represents the formation of bonding molecular orbital from the atomic orbitals having wave functions and
EASY
AP EAPCET
IMPORTANT
The total number of and bonds present in the following compound are
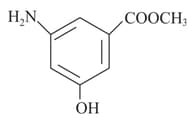
EASY
AP EAPCET
IMPORTANT
The geometry of molecule is
MEDIUM
AP EAPCET
IMPORTANT
The pair of xenon compounds, which have a same number of lone pairs of electrons on the central atom is
