HARD
9th Foundation
IMPORTANT
Earn 100
Observe the given flow chart and answer the following questions.
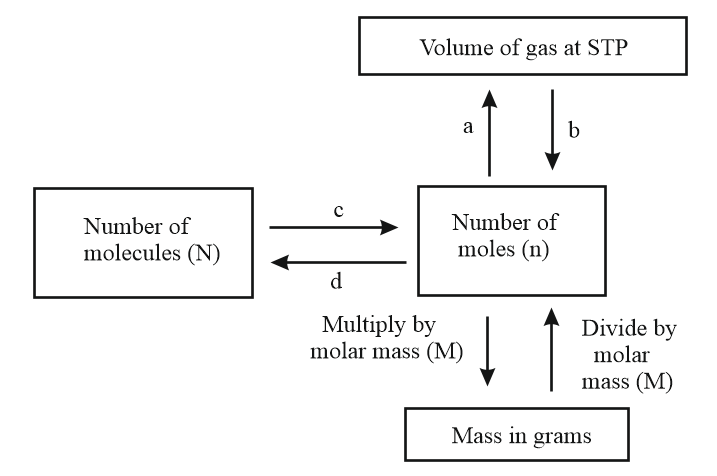
What are c and d?

(a)c= multiply by divide by
(b)c= multiply by multiply by
(c)c = divide by multiply by
(d)c = divide by divide by
50% studentsanswered this correctly

Important Questions on Atoms and Molecules
MEDIUM
9th Foundation
IMPORTANT
of water can be represented as
(i) mole of water
(ii) moles of water
(iii) molecules of water
(iv) molecules of water.
HARD
9th Foundation
IMPORTANT
(I) moles of and moles of have the same mass.
(II) of has more atoms than that present in of .
(III) of solid sulphur contains molecules.
HARD
9th Foundation
IMPORTANT
MEDIUM
9th Foundation
IMPORTANT
MEDIUM
9th Foundation
IMPORTANT
I Phosphate ion is a trivalent and positive ion.
II Calcium ion is trivalent and positive.
III Phosphorus is a polyatomic molecule.
IV Aluminium ion is divalent and positive.
MEDIUM
9th Foundation
IMPORTANT
Fill in the blanks by selecting the option with the correct sequence of words. The symbolic representation of a molecule showing actual number of various atoms present in it is called its X formula. The number of atoms of all elements present in a molecule is known as its Y. Z formula gives the simplest ratio of atoms present in one molecule of the compound.
| X | Y | Z | |
| (A) | Chemical | Valency | Molecular |
| (B) | Molecular | Valency | Chemical |
| (C) | Empirical | Atomicity | Molecular |
| (D) | Molecular | Atomicity | Empirical |
HARD
9th Foundation
IMPORTANT
HARD
9th Foundation
IMPORTANT
