EASY
Earn 100
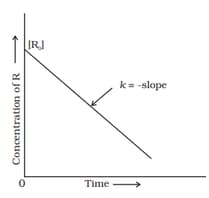
Observe the graph in the diagram and answer the given question:
How does the half-life of zero order reaction relate to its rate constant?

Important Questions on Chemical Kinetics
MEDIUM
Derive an expression of rate constant for zero order reaction.
MEDIUM
Which expression among the following is correct for a zero-order reaction and a first order reaction respectively? Here denotes the intial concentration of reactant:
MEDIUM
Starting from the integrated law of a zero-order reaction, , show that the half-life time of a reaction is directly proportional to the initial molar concentration of the reactant.
EASY
Write the mathematical relation between half life of zero order reaction and its rate constant.
MEDIUM
For a zero - order reaction with rate constant k, the slope of the plot reactant concentration against time is
MEDIUM
For a first-order reaction, prove that .
EASY
The decomposition of ammonia on a platinum surface is a zero-order reaction. If the rate constant is , how long will it take to reduce the initial concentration of ammonia from .
HARD
Draw the graph of half-life period versus initial concentration of reactant for a zero order reaction. Give reasons in favour of your answer.
EASY
In a zero order reaction, when is plotted against is obtained.
EASY
Rate constant for zero order reaction is if the concentration of the reactant after is What is the initial concentration of reactant?
HARD
Which of the following relation is correct for zero-order reaction?
EASY
The half-life for a zero order reaction having initial concentration of reactant is . The rate constant (in ) for the reaction is
HARD
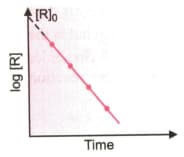
Examine the graph given below. Identify the integrated rate equation and the order of the reaction corresponding to it.
HARD
Determine the expression of rate constant for zero order reaction.
MEDIUM
The half-life of a zero order reaction is second. If the reaction takes second to complete, calculate in terms of .
EASY
The given graph is a representation of kinetics of a reaction.
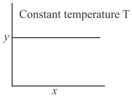
The and axes for zero and first order reactions, respectively are
HARD
Derive the integrated rate equation of Zero Order Reaction.
EASY
For zero order reaction, a plot of versus will be
MEDIUM
Under the same reaction conditions, initial concentration of of a substance becomes half in and through first order and zero order kinetics, respectively. Ratio of the rate of constants for first order and zero order of the reaction is
EASY
The rate constant of the reaction is mole per liter per second. If the initial concentration of A is 5 M, then the concentration of B after 20 minutes is: