EASY
10th CBSE
IMPORTANT
Earn 100
On adding a few drops of Universal indicator solution to three unknown colourless solutions and taken separately in three test tubes shown in the diagrams, a student observed the changes in colour as green in solution , red in solution and violet in solution . 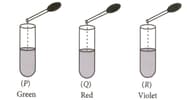
The decreasing order of of the three solutions is:

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Practical Chemistry
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
EASY
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
MEDIUM
10th CBSE
IMPORTANT
A student added acetic acid to test tubes I, II, III and IV containing the labelled substances and then brought a burning splinter near the mouth of each test tube.
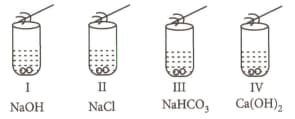
The splinter would be extinguished when brought near the mouth of test tube
