MEDIUM
AS and A Level
IMPORTANT
Earn 100
On analysis, a hydrocarbon was found to contain of carbon and of hydrogen. Calculate the empirical formula of the hydrocarbon.

Important Questions on Introduction to Organic Chemistry
HARD
AS and A Level
IMPORTANT
On analysis, a hydrocarbon was found to contain of carbon and of hydrogen. Further investigation showed that the relative molecular muss of the hydrocarbon was . Deduce its molecular formula.
MEDIUM
AS and A Level
IMPORTANT
A compound contains the elements carbon, hydrogen and oxygen. Its empirical formula is and its relative molecular mass is . Deduce the molecular formula of the compound.
MEDIUM
AS and A Level
IMPORTANT
Draw the displayed formula of ethene (molecular formula ).
MEDIUM
AS and A Level
IMPORTANT
Draw the displayed formula of propane (molecular formula ).
HARD
AS and A Level
IMPORTANT
Draw the skeletal formula of pentane, a straight-chain hydrocarbon with a molecular formula of .
MEDIUM
AS and A Level
IMPORTANT
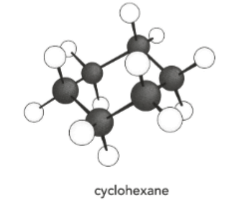
Draw the skeletal formula of the following molecule.
HARD
AS and A Level
IMPORTANT
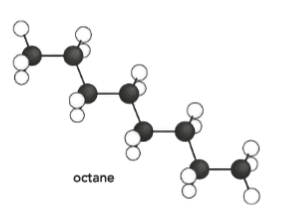
Draw the skeletal formula of the following molecule.
HARD
AS and A Level
IMPORTANT
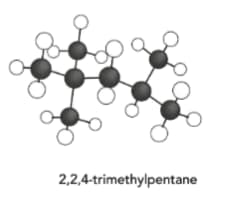
Draw the skeletal formula of the following molecule.