MEDIUM
JEE Main
IMPORTANT
Earn 100
On applying pressure to the equilibrium reaction, , which phenomenon will occur?
(a)More ice will be formed.
(b)More water will be formed.
(c)Equilibrium will not be disturbed.
(d)Water will evaporate.
62.5% studentsanswered this correctly

Important Questions on Equilibrium
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
The graph given below relates for a reaction. The reaction must be
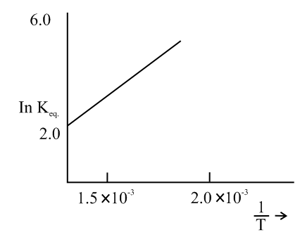
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
