EASY
JEE Main
IMPORTANT
Earn 100
On heating, lead (ll) nitrate gives a brown gas (A). The gas (A) on cooling changes to a colourless solid/liquid (B). (B) on heating with NO changes to ablue solid (C). The oxidation number of nitrogen in solid (C) is:
(a)
(b)
(c)
(d)
44.44% studentsanswered this correctly

Important Questions on Qualitative Analysis
EASY
JEE Main
IMPORTANT
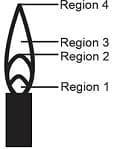
The hottest region of Bunsen flame shown in the figure below is:
EASY
JEE Main
IMPORTANT
Match List - I with List - II :
| List - I (Metal ion) | List - II (Group in Qualitative analysis) | ||
| (a) | (i) | Group - III | |
| (b) | (ii) | Group - IIA | |
| (c) | (iii) | Group - IV | |
| (d) | (iv) | Group - IIB |
MEDIUM
JEE Main
IMPORTANT
The sodium salt of an organic acid produces effervescence with concentrated . reacts with the acidified aqueous solution to give a white precipitate which decolourises acidic solution of . is
EASY
JEE Main
IMPORTANT
To an aqueous solution containing ions such as and was added , followed by . The total number of cations precipitated during this reaction is/are :
HARD
JEE Main
IMPORTANT
Acidic ferric chloride solution on treatment with excess of potassium ferrocyanide gives a Prussian blue coloured colloidal species. It is:
MEDIUM
JEE Main
IMPORTANT
For the following Assertion and Reason, the correct option is
Assertion (A) : When (II) and sulphide ions are mixed, they react together extremely quickly to give a solid.
Reason (R) : The equilibrium constant of is high because the solubility product is low.
MEDIUM
JEE Main
IMPORTANT
The incorrect statement is:
MEDIUM
JEE Main
IMPORTANT
A white sodium salt dissolves readily in water to give a solution which is neutral to litmus. When silver nitrate solution is added to the before mentioned solution, a white precipitate is obtained which does not dissolve in dilute nitric acid. The anion is:
