MEDIUM
JEE Main
IMPORTANT
Earn 100
On increasing the pressure, in which direction will the gas phase reaction proceed to re-establish, (as predicted by applying the Le Chatelier's principle) for the reaction: . Which of the following options is correct, if the total pressure at which the equilibrium is established, is increased without changing the temperature?
(a) will remain same.
(b) will decrease.
(c) will increase.
(d)
50% studentsanswered this correctly

Important Questions on Chemical Equilibrium
EASY
JEE Main
IMPORTANT
For the equilibrium reaction , which of the following statements is true?
EASY
JEE Main
IMPORTANT
The equilibrium reaction is attained at in a closed container and an inert gas, helium, is introduced. Which of the following statement(s) is/are correct?
EASY
JEE Main
IMPORTANT
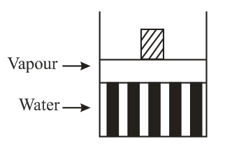
Some quantity of water is contained in a container as shown as shown in figure. As neon is added to this system at constant pressure, the amount of liquid water in the vessel
EASY
JEE Main
IMPORTANT
The equilibrium in aqueous medium at shifts towards the left in the presence of which of the following?
MEDIUM
JEE Main
IMPORTANT
The thermal dissociation equilibrium reaction of is studied under different conditions
For this equilibrium reaction, the correct statement(s) is/ (are):
MEDIUM
JEE Main
IMPORTANT
When is heated in a closed vessel, oxygen is liberated and is left behind. At equilibrium ,
HARD
JEE Main
IMPORTANT
An industrial fuel, 'water gas', which consists of a mixture of and can be made by passing steam over red-hot carbon. The reaction is
The yield of and at equilibrium would be shifted to the product side by:
MEDIUM
JEE Main
IMPORTANT
The reactions in which the yield of the products cannot be increased by applying high pressure are
