EASY
Earn 100
On the top of a mountain water boils at
(a)High temperature
(b)Same temperature
(c)High Pressure
(d)Low temperature
50% studentsanswered this correctly
Important Questions on Solutions
HARD
HARD
EASY
MEDIUM
Which of the following relations is correct?
MEDIUM
The vapour pressure vs. temperature curve for a solution solvent system is shown below.
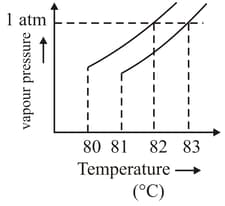
The boiling point of the solvent is _____°C.
EASY
MEDIUM
EASY
MEDIUM
Statement - : Equilibrium constant does not change unless temperature is changed.
EASY
MEDIUM
A liquid is kept in a closed vessel. If a glass plate of negligible mass with small hole is kept on top of the liquid surface, then vapour pressure of the liquid in the vessel is:
EASY
EASY
The pair of boiling point and compound are given as,
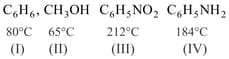
Which will show lowest vapour pressure at room temperature?
EASY
EASY
Statement - 2: In the presence of a more volatile liquid solute, only solute will form the vapours and solvent will not.
EASY
EASY
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
EASY
EASY
MEDIUM

