HARD
Earn 100
On which of the following parameters does the rate constant of a reaction depend?
(i) time, (ii) concentration of reactants, (iii) temperature, and (iv) catalyst
(a)(i) and (ii) only
(b)(iii) and (iv) only
(c)(iii) only
(d)(i), (ii), (iii) and (iv)
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
EASY
EASY
(a) In which test tube does the reaction proceed faster?
(b) Give reason.
(c) Give an instance in daily life, where such condition is made use.
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
HARD
For an elementary chemical reaction, , the expression for is:
MEDIUM
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
EASY
HARD
MEDIUM
Which one of the following statements is correct?
HARD
(Assume that all these gases behave as ideal gases)
MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
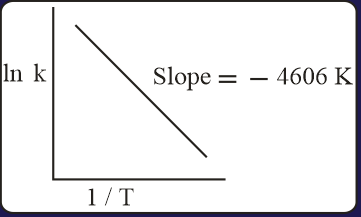
EASY
EASY
EASY

