HARD
Earn 100
One mole of a diatomic ideal gas is taken through a cyclic process starting from point A. The process A → B is an adiabatic compression. B → C is isobaric expansion, C → D an adiabatic expansion and D → A is isochoric respectively.
The volume ratios are given to be and and the temperature at A is . The efficiency of the cyclic process (in ) is . Find value of where is the greatest integer function.
50% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
MEDIUM
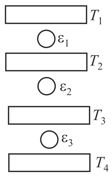
Three Carnot engines operate in series between a heat source at a temperature and a heat sink at temperature (see figure). There are two other reservoirs at temperature and as shown, with The three engines are equally efficient if:
MEDIUM
A Carnot engine has an efficiency of When the temperature of the sink is reduced by , its efficiency is doubled. The temperatures of the source and the sink are, respectively,
HARD
Two ideal Carnot engines operate in a cascade (all heat given up by one engine is used by the other engine to produce work) between temperatures, and . The temperature of the hot reservoir of the first engine is and the temperature of the cold reservoir of the second engine is . is the temperature of the sink of the first engine which is also the source for the second engine. How is related to and , if both the engines perform an equal amount of work?
EASY
A refrigerator works between and . It is required to remove calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is:
(Take cal = Joules)
EASY
A Carnot's engine having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is the amount of energy absorbed from the reservoir at lower temperature is
EASY
If minimum possible work is done by a refrigerator in converting 100 grams of water at to ice, how much heat (in calories) is released to the surroundings at temperature (Latent heat of ice) to the nearest integer?
EASY
A Carnot engine having an efficiency of is being used as a refrigerator. If the work done on the refrigerator is the amount of heat absorbed from the reservoir at a lower temperature is
MEDIUM
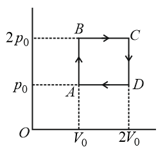
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
EASY
The coefficient of performance of a refrigerator is . If the temperature inside the freezer is , the temperature of the surrounding to which it rejects heat is:
MEDIUM
A Carnot freezer takes heat from water at inside it and rejects it to the room at a temperature of The latent heat of ice is If 5kg of water at is converted into ice at by the freezer, then the energy consumed by the freezer is close to :
EASY
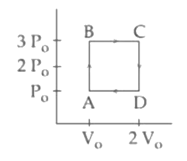
An engine operates by taking a monatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to
EASY
A Carnot engine, having an efficiency of as heat engine, is used as a refrigerator. If the work done on the system is , the amount of energy absorbed from the reservoir at a lower temperature is:
EASY
The efficiency of a Carnot engine depends upon
MEDIUM
A heat engine is involved with exchange of heat of and , during one cycle achieving and efficiency of . The value of is:
MEDIUM
A reservoir is at and Carnot's engine takes a thousand of heat from it and exhausts it to a sink at . What is the amount of work and the efficiency of the engine?
EASY
Two carnot engines and are connected in series in such a way that the work outputs are equal when the temperatures of hot and cold reservoirs of are and , and of engine are and , respectively. Then the temperature is
EASY
An air conditioner removes heat at a rate of from a room. A power of is required to run the . The coefficient of performance of the is of that of the refrigerator operating between outside and room temperature. If outside temperature is , what will be room temperature?
EASY
A Carnot engine operates between two reservoirs of temperatures and . The engine performs of work per cycle. The heat energy (in ) delivered by the engine to the low temperature reservoir, in a cycle, is _____________
EASY
The temperature inside a refrigerator is and the room temperature is . The amount of heat delivered to the room for each joule of electrical energy consumed ideally will be
EASY
Two carnot engines and are operated in series. The first one, receives heat at and rejects to a reservoir at temperature The second engine receives heat rejected by the first engine and, in turn, rejects to a heat reservoir at Calculate the temperature if the work outputs of the two engines are equal:

