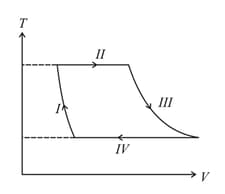
One mole of a monatomic ideal gas undergoes a cyclic process as shown in the figure (where is the volume and is the temperature). Which of the statements below is (are) true ?


Important Questions on Thermodynamics
Two rigid boxes containing different ideal gases are placed on a table. The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Box A contains one mole of nitrogen at temperature , while box contains one mole of helium at temperature . Then the final temperature of the gases, in terms of is
The work of is performed in order to compress one-kilo mole of gas adiabatically and in this process the temperature of the gas increases by The gas is
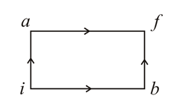
When a system is taken from state to state along the path, it is found that and . Along the path along the path is
A diatomic ideal gas is used in a Carnot engine as the working substance. If, during the adiabatic expansion part of the cycle the volume of the gas increases from to , the efficiency of the engine is :
