MEDIUM
Earn 100
One mole of a mono-atomic gas behaving as per at is subjected to reversible isoentropic compression untill final temperature reaches . If the initial pressure was then the value of (final) is -
(given )
(a)1.75
(b)0.176
(c)1.0395
(d)2.0
66.67% studentsanswered this correctly
Important Questions on Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
One mole of an ideal monoatomic gas undergoes two reversible processes and as shown in the given figure:
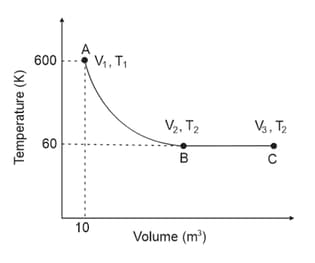
is an adiabatic process. If the total heat absorbed in the entire process and is , the value of is
[Use molar heat capacity of the gas at constant pressure, ]
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(Given is gas constant)
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
Reversible process
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
Two moles of an ideal monoatomic gas occupies a volume at . The gas expands adiabatically to a volume . Calculate the final temperature of the gas and change in its internal energy.
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 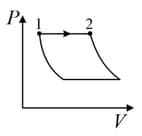 |
| (II) | (ii) Isochoric | (Q) 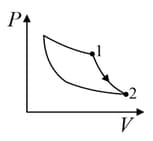 |
| (III) | (iii) Isobaric | (R) 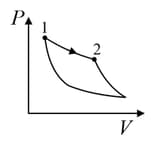 |
| (IV) | (iv) Adiabatic | (S) 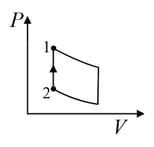 |
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 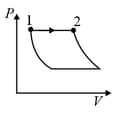 |
| (II) | (ii) Isochoric | (Q) 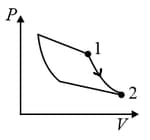 |
| (III) | (iii) Isobaric | (R) 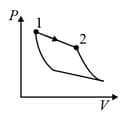 |
| (IV) | (iv) Adiabatic | (S) 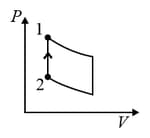 |
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
MEDIUM
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
HARD
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other
(: pressure, : volume, : temperature, : enthalpy, : entropy)
EASY
Physical Sciences>Matter and Its Interactions>Develop a model that predicts and describes changes in particle motion, temperature, and state of a pure substance when thermal energy is added or removed.>Structure and Properties of Matter - Gases and liquids are made of molecules or inert atoms that are moving about relative to each other

