EASY
JEE Main
IMPORTANT
Earn 100
One mole of a monoatomic gas is carried along process as shown in the diagram. Find the net work done by gas.
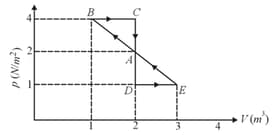

(a)
(b)
(c)
(d)
(e)
50% studentsanswered this correctly

Important Questions on The First Law of Thermodynamics
EASY
JEE Main
IMPORTANT
One mole of an ideal gas with heat capacity at constant pressure undergoes the process where, and are constants. If its volume increases from to the amount of heat transferred to the gas is
EASY
JEE Main
IMPORTANT
Ideal monoatomic gas is taken through a process The molar heat capacity for the process is___ (where, is heat supplied and is change in internal energy)
MEDIUM
JEE Main
IMPORTANT
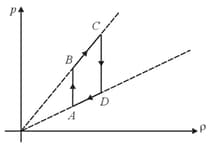
Pressure versus density graph of an ideal gas is shown in figure
MEDIUM
JEE Main
IMPORTANT
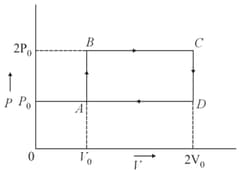
moles of a monoatomic gas is carried round the reversible rectangular cycle as shown in the diagram. The temperature at is . The thermodynamic efficiency of the cycle is approximately
EASY
JEE Main
IMPORTANT
A closed system undergoes a change of state by process for which and The system is now returned to its initial state by a different path for which is . The work done by the gas in the process is
EASY
JEE Main
IMPORTANT
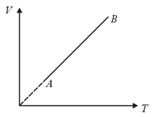
An ideal monoatomic gas undergoes the process AB as shown in the figure. If the heat supplied and the work done in the process are and respectively. The ratio is
HARD
JEE Main
IMPORTANT
One mole of a gas expands with temperature such that its volume, where is a constant. If the temperature of the gas changes by then the work done by the gas is
HARD
JEE Main
IMPORTANT
moles of an ideal gas undergo a process in which the temperature changes with volume as . The work done by the gas as the temperature changes from to is
