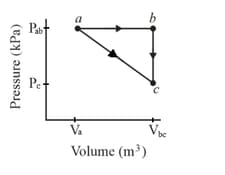
One mole of an ideal diatomic gas goes from to along the diagonal path as shown. The scale of the vertical axis is set by and and the scale of the horizontal axis is set by and . During the transition,
what is the change in the internal energy of the gas
how much energy is added to the gas as heat?
how much heat is required if the gas goes from to along the indirect path ?


Important Questions on The Kinetic Theory of Gases
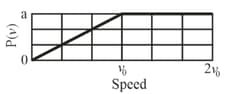
Given figure shows a hypothetical speed distribution for a sample of gas particles (Note that for speed ). What are the values of
(a)
(b)
(c) ?
(d) What fraction of the particles have a speed between and ?
(a) average and
(b) rms speeds.
(c) Is
At and pressure, the mean free paths for argon gas and nitrogen gas are and
(a) Find the ratio of the diameter of an atom to that of an molecule. What is the mean free path of argon at
(b) and , and
(c) and ?
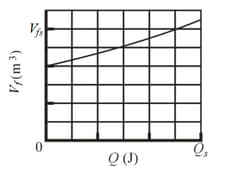
Suppose of an ideal gas undergoes an isothermal expansion as energy is added to it as heat . If the given figure shows the final volume versus what is the gas temperature? The scale of the vertical axis is set by , and the scale of the horizontal axis is set by (Use )
What is the average translational kinetic energy of nitrogen molecules at
Given:- Boltzmann constant, .
