MEDIUM
Earn 100
One mole of an ideal gas expands adiabatically from an initial state to final state . Another mole of the same gas expands isothermally from a different initial state to the same final state . The ratio of the specific heats at constant pressure and constant volume of this ideal gas is . What is the ratio ?
(a)
(b)
(c)
(d)
80.46% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then:
HARD
A mixture of ideal gas containing moles of monatomic gas and mole of rigid diatomic gas is initially at pressure volume and temperature If the gas mixture is adiabatically compressed to a volume , then the correct statement(s) is/are,
(Given is gas constant)
MEDIUM
If a monoatomic gas is compressed adiabatically to th of its initial volume, then its pressure becomes
HARD
Consider one mole of helium gas enclosed in a container at initial pressure and volume . It expands isothermally to volume . After this, the gas expands adiabatically and its volume becomes . The work done by the gas during isothermal and adiabatic expansion processes are and , then is _________ .
MEDIUM
The bulk modulus of a gas is defined as, . For an adiabatic process, the variation of is proportional to . For an ideal gas, is
EASY
In an adiabatic process, the density of a diatomic gas becomes times its initial value. The final pressure of the gas is found to be times the initial pressure. The value of is:
EASY
litre of dry air at STP expands adiabatically to a volume of litres. If the work done by air is: [Take air to be an ideal gas]
EASY
One mole of ideal gas goes through a process constant, where and are pressure and volume respectively. Let be the work done by the gas as its temperature is increased by . The value of is ( is the universal gas constant)
MEDIUM
Two moles of an ideal monoatomic gas occupies a volume at . The gas expands adiabatically to a volume . Calculate the final temperature of the gas and change in its internal energy.
HARD
An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state to state . denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas is .
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 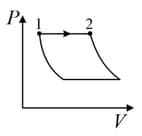 |
| (II) | (ii) Isochoric | (Q) 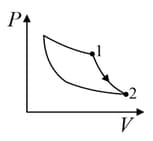 |
| (III) | (iii) Isobaric | (R) 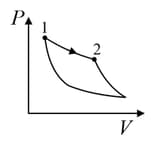 |
| (IV) | (iv) Adiabatic | (S) 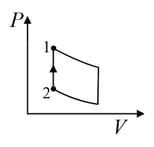 |
EASY
An ideal monoatomic gas at expands adiabatically to twice its volume. The final temperature of gas is
MEDIUM
One mole of an ideal monatomic gas undergoes an adiabatic expansion in which its volume becomes eight times its initial value. If the initial temperature of the gas is and the universal gas constant , then how much is the decrease in its internal energy (in ) ?
EASY
When a tyre pumped to a pressure at suddenly bursts, find its final temperature
HARD
An engine takes in moles of air at and , and compresses it adiabatically to of the original volume. Assuming air to be a diatomic ideal gas made up of rigid molecules, the change in its internal energy during this process comes out to be . The value of to the nearest integer is:
HARD
The rapid changes in pressure and volume of an ideal gas under thermal isolation are governed by constant. The gas may be ___ .
EASY
For an ideal gas with initial pressure and volume and respectively, a reversible isothermal expansion happens, when its volume becomes . Then, it is compressed to its original volume by a reversible adiabatic process. If the final pressure is then which of the following statement(s) is/are true?
HARD
A gas consisting of rigid molecules expands adiabatically such that r.m.s. speed of its molecules becomes half. The ratio of final and initial volumes of the gas is (ratio of specific heat capacities of the gas is )
HARD
An ideal gas is undergoing a cyclic thermodynamic process in different ways as shown in the corresponding P-V diagrams in column 3 of the table. Consider only the path from state 1 to state 2. W denotes the corresponding work done on the system. The equations and plots in the table have standard notations as used in thermodynamic process. Here is the ratio of heat capacities at constant pressure and constant volume. The number of moles in the gas is .
| Column – 1 | Column – 2 | Column – 3 |
| (I) | (i) Isothermal | (P) 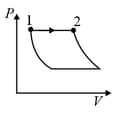 |
| (II) | (ii) Isochoric | (Q) 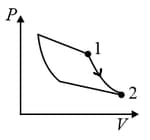 |
| (III) | (iii) Isobaric | (R) 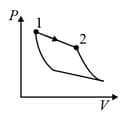 |
| (IV) | (iv) Adiabatic | (S) 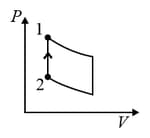 |
HARD
The heat capacity of one mole an ideal is found to be where is constant. The equation obeyed by this gas during a reversible adiabatic expansion is:
HARD
Consider a spherical shell of radius R at temperature T. The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume and pressure . If the shell now undergoes an adiabatic expansion the relation between T and R is:

