EASY
NEET
IMPORTANT
Earn 100
One mole of an ideal gas goes from an initial state A to final state B via two processes. It firstly undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct P-V diagram representing the two processes is :-
(a)
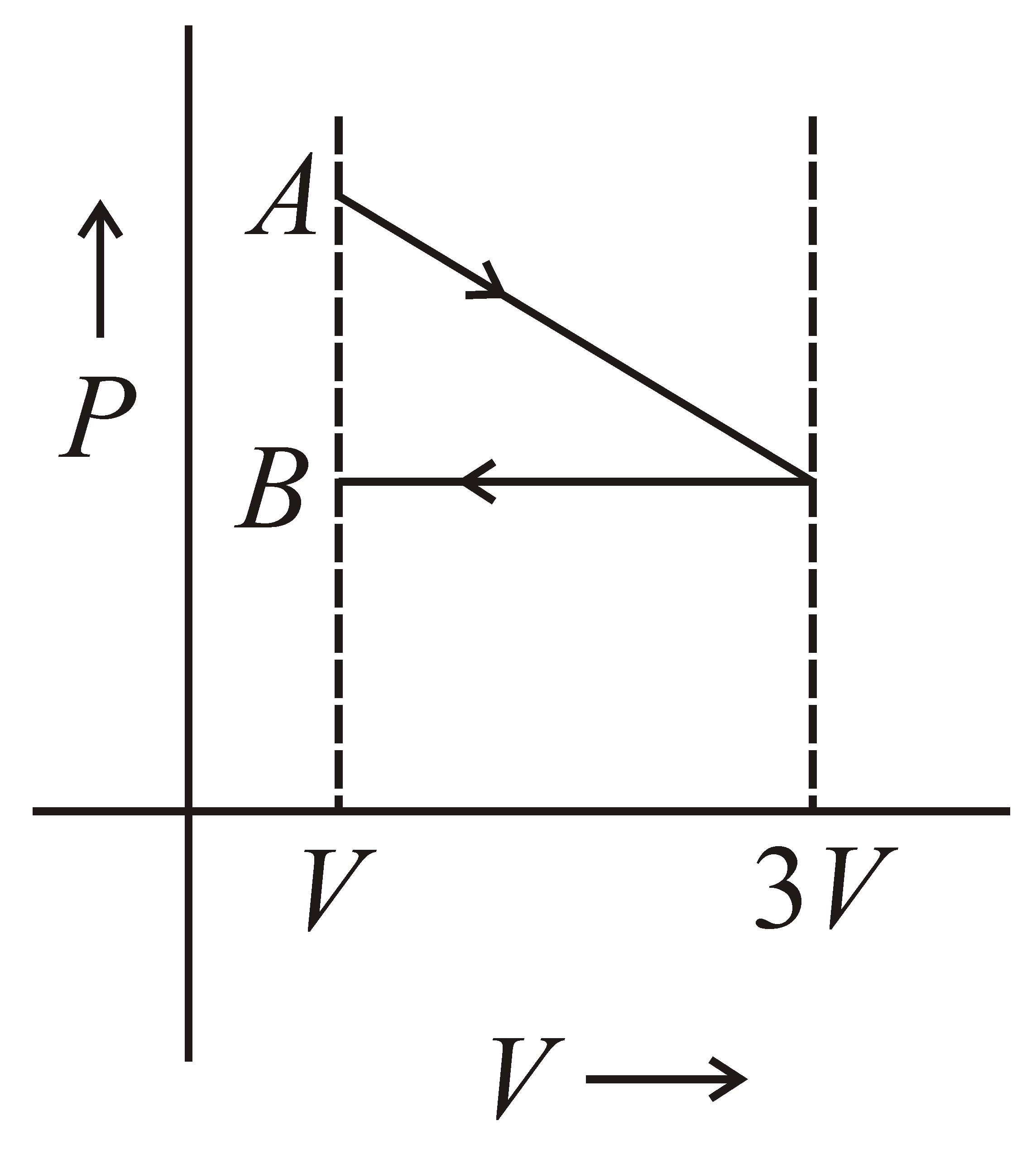
(b)
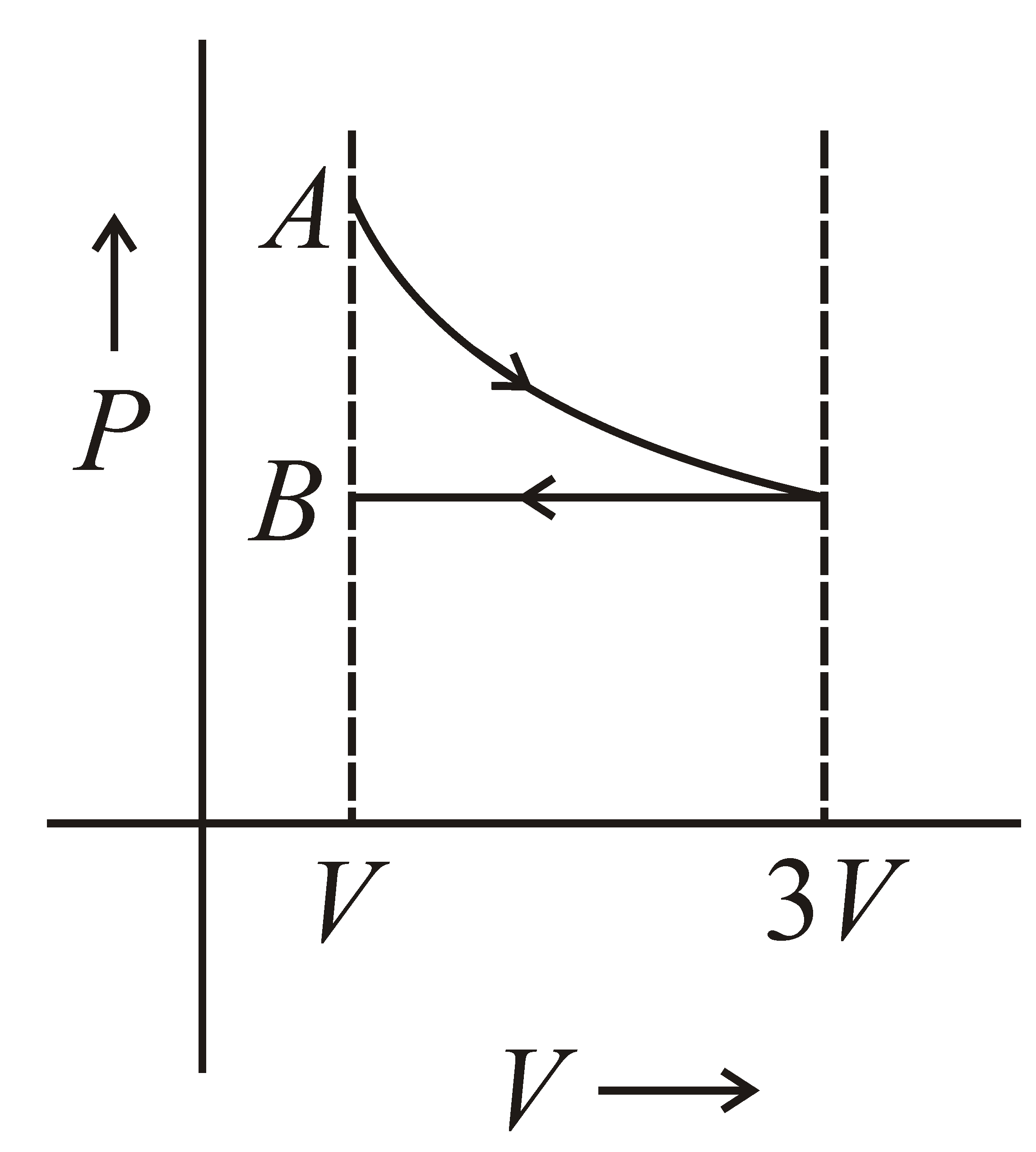
(c)
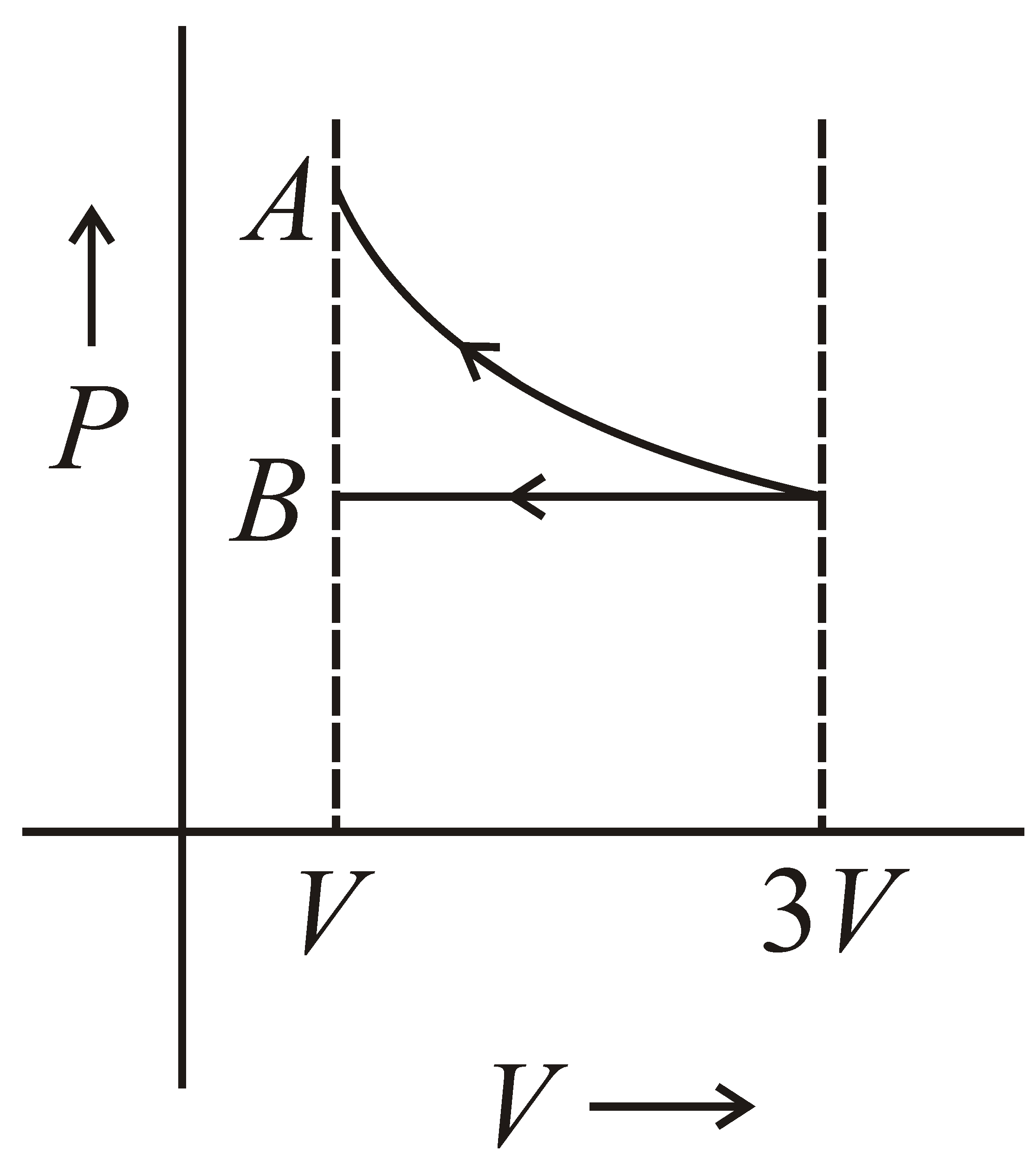
(d)
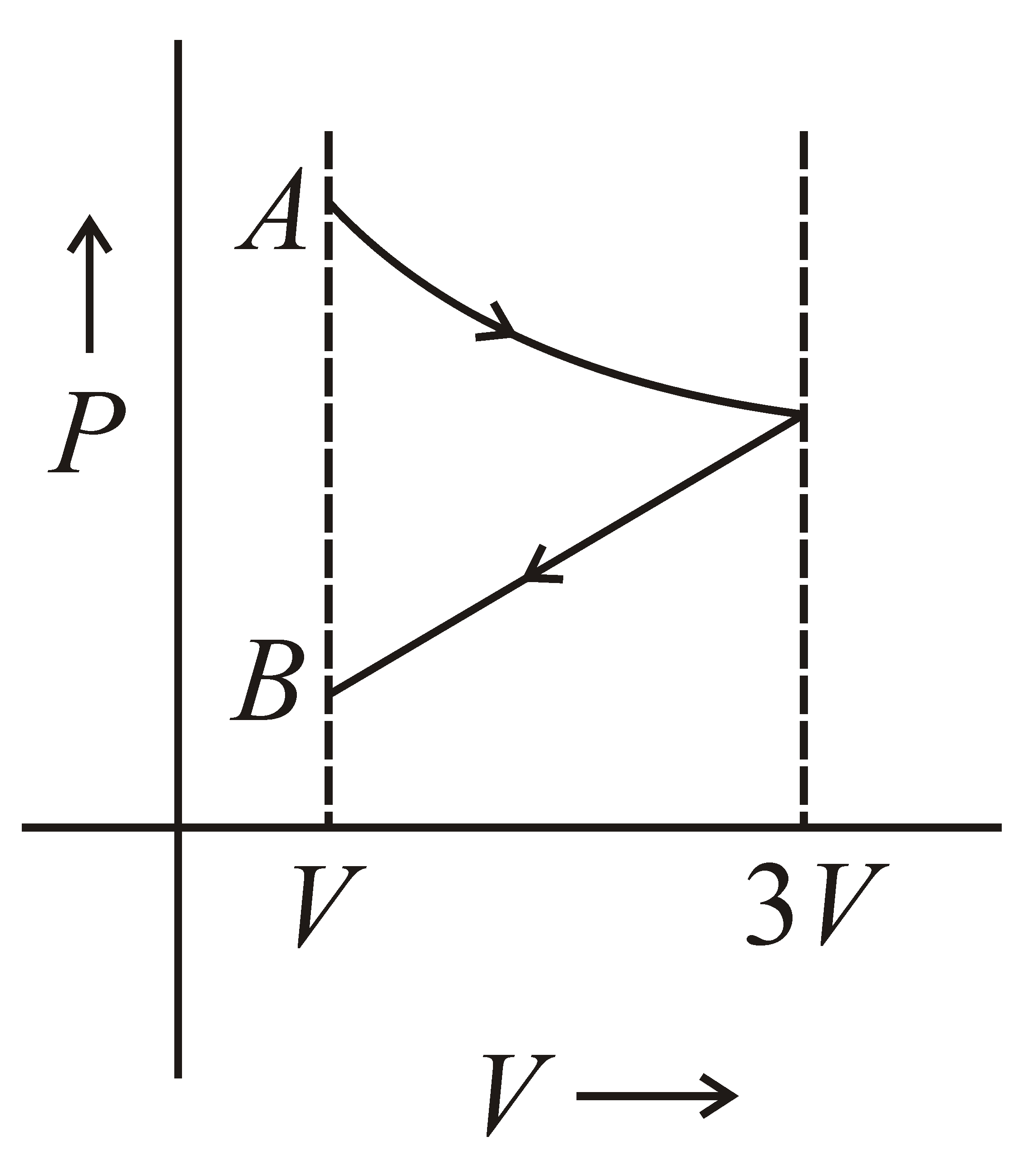
100% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
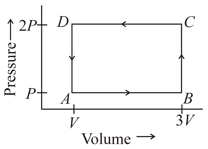
A thermodynamic system is taken through the cycle. ABCD as shown in figure. Heat rejected by the gas during the cycle is:-
EASY
NEET
IMPORTANT
An ideal gas goes from state A to state B via three different processes as indicated in the P-V diagram.
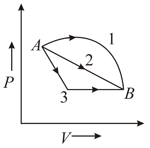
If Q1, Q2, Q3 indicate the heat absorbed by the gas along the tree processes and indicate the change in internal energy along the three processes respectively, then:-
EASY
NEET
IMPORTANT
Conversion of water to steam is accompanied by which process :-
EASY
NEET
IMPORTANT
What is the slope for an isothermal process in PV indicator diagram:-
MEDIUM
NEET
IMPORTANT
The molar specific heats of an ideal gas at constant pressure and volume are denoted by and , respectively. If and is the universal gas constant, the is equal to
MEDIUM
NEET
IMPORTANT
During an adiabatic process the pressure of a gas is found to be proportional to the cube of its absolute temperature. The ratio for the gas is
EASY
NEET
IMPORTANT
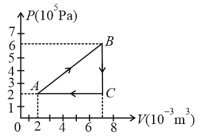
A gas is taken through the cycle as shown. What is the net work done by the gas ?
MEDIUM
NEET
IMPORTANT
A gas mixture contains one mole gas and one mole gas, the ratio of specific heat at constant pressure to that at constant volume of the mixture is
