HARD
Physics
IMPORTANT
Earn 100
One mole of an ideal monatomic gas undergoes a linear process from to , in which is pressure and its volume change as shown in figure. The maximum temperature of the gas during this process is
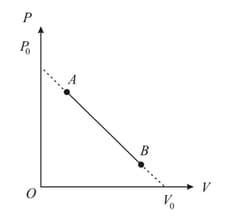
(a)
(b)
(c)
(d)
40% studentsanswered this correctly

Important Questions on Kinetic Theory of Gases
HARD
Physics
IMPORTANT
Molar heat capacity of an ideal gas in the process PVx = constant is given by
An ideal diatomic gas with occupies a volume V1 at a pressure P1 . The gas undergoes a process in which the pressure is proportional to the volume. At the end of the process the rms speed of the gas molecules has doubled from its initial value.
Heat supplied to the gas in the given process is
HARD
Physics
IMPORTANT
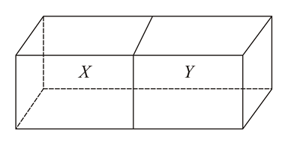
Container shown has two identical sections with a common wall between them which does not allow gas to leak through. Both sections contain helium gas with in compartment and in compartment The two halves of the container are at the same temperature. Which of the following is the same for the gas in the two section and
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
HARD
Physics
IMPORTANT
EASY
Physics
IMPORTANT
