MEDIUM
Earn 100
One mole of an ideal monoatomic gas is taken round the cyclic process ABCA as shown in fig. Calculate
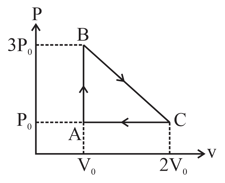
(a) The work done by the gas.
b) The heat rejected by the gas in the path and heat absorbed in the path .
(c) The net heat absorbed by the gas in the path .

Important Questions on Chemical Thermodynamics
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
HARD
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
EASY
HARD
EASY
EASY
EASY
EASY

