MEDIUM
JEE Main
IMPORTANT
Earn 100
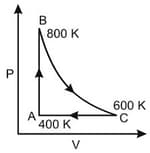
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :

50% studentsanswered this correctly
Important Questions on Thermodynamics
HARD
JEE Main
IMPORTANT

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
JEE Main
IMPORTANT
A Carnot engine whose heat sinks is at , has an efficiency of . By how many degrees should the temperature of the source be changed to increase the efficiency by of the original efficiency ?
MEDIUM
JEE Main
IMPORTANT
A Carnot engine takes of heat from a reservoir at and gives heat to a sink at . The work done by the engine is
EASY
JEE Main
IMPORTANT
A monoatomic gas performs a work of , where is the heat supplied to it. The molar heat capacity of the gas will be _____ during this transformation, where is the gas constant.
MEDIUM
JEE Main
IMPORTANT
The ratio of specific heats in terms of degree of freedom is given by :
EASY
JEE Main
IMPORTANT
A heat engine operates with the cold reservoir at temperature . The minimum temperature of the hot reservoir, if the heat engine takes heat from the hot reservoir and delivers heat to the cold reservoir per cycle, is _____ .
EASY
JEE Main
IMPORTANT
The efficiency of a Carnot's engine, working between steam point and ice point, will be
MEDIUM
JEE Main
IMPORTANT
A thermally insulated vessel contains an ideal gas of molecular mass and ratio of specific heats . Vessel is moving with speed and is suddenly brought to rest. Assuming no heat is lost to the surrounding and vessel temperature of the gas increases by :
( universal gas constant)
