MEDIUM
NEET
IMPORTANT
Earn 100
Osmotic pressure of a solution of glucose is same as solution of a non-volatile non-electrolyte solute. The molar mass of the solute is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solutions
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
Colligative properties have many practical uses, some of them may be:
I: Melting of snow by salt.
II: Desalination of seawater.
III: Determination of molar mass.
IV: Determination of melting point and boiling point of solvent actual practical uses are:
EASY
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
Consider the following terms (molality):
Terms which can be expressed in degree (temperature) are
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
What is the normal boiling point of the solution represented by the phase diagram?
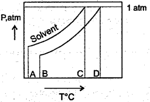
MEDIUM
NEET
IMPORTANT
