MEDIUM
JEE Main
IMPORTANT
Earn 100
Osmotic pressure of solution of glucose is and that of solution of cane sugar is . The osmotic pressure of the mixture containing equal volumes of the two solutions will be:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Solutions
HARD
JEE Main
IMPORTANT
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
on reaction with in aqueous solution gives blue colour. These are separated by a semipermeable membrane AB as shown. Due to osmosis, there is
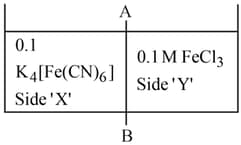
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
solution of is isotonic with solution of urea at the same temperature. The degree of dissociation of is
HARD
JEE Main
IMPORTANT
MEDIUM
JEE Main
IMPORTANT
