EASY
JEE Main/Advance
IMPORTANT
Earn 100
Out of and which has higher bond angle and why?

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main/Advance
IMPORTANT
The bond length is and bond length is . If electronegativities of and are and , respectively, the bond length is likely to be:
MEDIUM
JEE Main/Advance
IMPORTANT
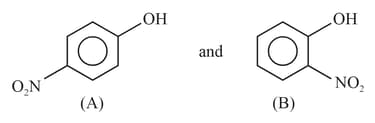
Out of the two compounds shown below, the vapour pressure of at a particular temperature is expected to be
EASY
JEE Main/Advance
IMPORTANT
The structure of can be best described as :-
EASY
JEE Main/Advance
IMPORTANT
The correct increasing bond angle among and follows the order :-
EASY
JEE Main/Advance
IMPORTANT
How many sigma and pi bonds are present in tetracyanoethylene ?
MEDIUM
JEE Main/Advance
IMPORTANT
The types of bond present in are:
EASY
JEE Main/Advance
IMPORTANT
How many bonded electron pairs are present in molecule?
MEDIUM
JEE Main/Advance
IMPORTANT
When and orbitals overlap, the bond strength decreases in the order :-
