EASY
Earn 100
Oxidation of glycerol with conc. gives mainly glyceric acid.
(a)True
(b)False
50% studentsanswered this correctly
Important Questions on Hydroxy Compounds and Ethers
MEDIUM
MEDIUM
Identify to which type does the following complex reactions belongs to-
- dehydration of 2-methyl-2-butanol
MEDIUM
Complete the reaction and write the name of the product.
MEDIUM
EASY
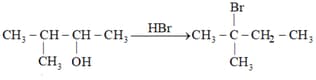
Give a mechanism for this reaction.
EASY
EASY
EASY
EASY
EASY
EASY
EASY
Oxidation of a primary alcohol to carboxylic acid.
EASY
EASY
EASY
EASY
MEDIUM
HARD
EASY
EASY

