MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
Oxygen, nitrogen and helium gas are kept in three identical adiabatic containersand , respectively at equal pressure. When the gases are pushed to half their original volumes. (initial temperature is same)
16.67% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
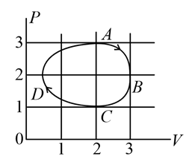
The figure shows the plot of an ideal gas taken through a cycle . The part is a semi-circle and is half of an ellipse. Then,
MEDIUM
JEE Main/Advance
IMPORTANT
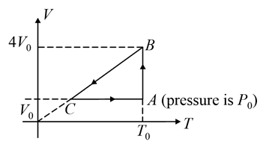
One mole of an ideal gas in initial state A undergoes a cyclic process , as shown in the figure. Its pressure at is . Choose the correct option(s) from the following :