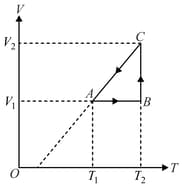
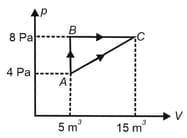
diagram of an ideal gas for a process is as shown in the figure.
Find the change in internal energy of the gas during the process .
Find total heat absorbed or released by the gas during the process .
Plot pressure versus density graph of the gas for the process .

Find total heat absorbed or released by the gas during the process .
Plot pressure versus density graph of the gas for the process .

Important Questions on Laws of Thermodynamics
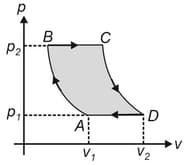
In the given graph, an ideal gas changes its state from to by two paths and .
Find the path along which work done is less.
The internal energy of gas at is and the amount of heat supplied in path is . Calculate the internal energy of the gas at .
The internal energy of gas at state is . Find the amount of heat supplied to the gas to go from to .
When a gas expands along , it does of work and absorbs of heat. When the gas expands along , it does of work and absorbs of heat.
How much heat does the gas exchange along ?
When the gas makes a transition from to along , of work is done on it from to . How much heat does it exchange along ?
What is the temperature of the gas?
The gas undergoes an adiabatic compression until its volume is decreased to . What is the new gas temperature?
How much work is done on the gas during the compression?
What is the change in the internal energy of the gas?
How much mechanical energy is dissipated in the collision?
Assuming that for the bob plus the bullet is , calculate the temperature increase of the system due to the collision. Take the molecular mass of the system to be .
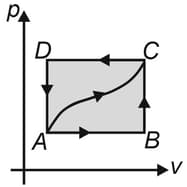
An ideal gas is carried through a thermodynamic cycle consisting of two isobaric and two isothermal processes, as shown in the figure. Show that the net work done in the entire cycle is given by the equation. .
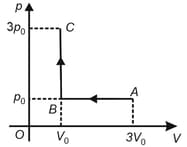
A cyclic process for of an ideal gas is shown in the diagram. The work done in and , respectively, is