EASY
Earn 100
Peroxy linkage is present in :-
(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Chemical Bonding & Molecular Structure
MEDIUM
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
The bond lengths (in of and are:
HARD
bond length in is than the bond length in . The bond length in is than that of the bond in . Choose the correct option for and from the given below.
MEDIUM
EASY
How many ions of the following have a bond order of
EASY
EASY
EASY
(A) and the other as Reason (R). Examine both the statements carefully and mark the correct choice.
(A) The bond angle in is greater than bond angle in
(R) Due to larger size of , hydrogen bonding does not occur in
MEDIUM
MEDIUM
What will be the CORRECT order of the C-F bond distance among the following?
and
EASY
MEDIUM
EASY
EASY
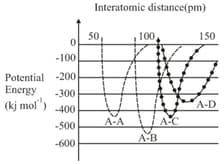
MEDIUM

