EASY
JEE Advanced
IMPORTANT
Earn 100
Peroxyacetic acid () is weaker acid than acetic acid () since-
(a)Negative charge in 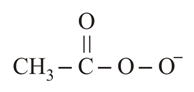 cannot be delocalized into the carbonyl group
cannot be delocalized into the carbonyl group
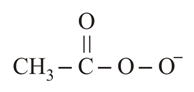 cannot be delocalized into the carbonyl group
cannot be delocalized into the carbonyl group(b)
group in shows effect
(c)Both are correct
(d)None is correct
50% studentsanswered this correctly

Important Questions on General Organic Chemistry
MEDIUM
JEE Advanced
IMPORTANT
in the increasing values.
EASY
JEE Advanced
IMPORTANT
Basic strength of
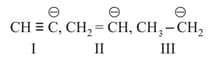
will be in order -
MEDIUM
JEE Advanced
IMPORTANT
Which of the following shows the correct order of decreasing acidity?
EASY
JEE Advanced
IMPORTANT
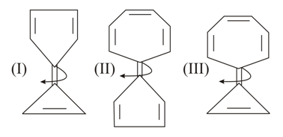
Compare carbon-carbon bond rotation across.
HARD
JEE Advanced
IMPORTANT
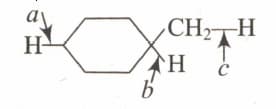
Arrange, in decreasing order of bond dissociation energies of the bonds, indicated with the arrows in following compound.
HARD
JEE Advanced
IMPORTANT
Three carboxylic acids namely benzoic acid, salicylic acid and -Dihydroxy benzoic acid are taken, then, the degree of dissociation of_______.
MEDIUM
JEE Advanced
IMPORTANT
The bond angles in methane, ammonia and methyl amine increase in the order-
EASY
JEE Advanced
IMPORTANT
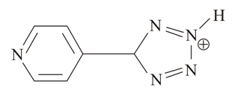
How many lone pairs are present in the given cation?